WHY AIDS POLICY NEEDS TO BE AUDITED /
REEXAMINED
38 REASONS
See the numbers in curly brackets: {n}
There are many, many more – these are just a few of the concerns raised by scientists and medical researchers.
Note: In the web page, footnotes that give
references are shown in square brackets: [n].
DEFINITIONS
The acronym “AIDS” means “Acquired Immune Deficiency Syndrome”. AIDS is not a single disease – that’s what “syndrome” means. Rather, it is any of some thirty long known illnesses, together with an indication of a retrovirus, known as HIV, in the body.
For instance:
Pneumonia + HIV = AIDS [incurable]
Pneumonia – HIV = Pneumonia [curable]
There have been several redefinitions of AIDS; each has expanded the conditions included under the AIDS umbrella.
WHAT IS AIDS POLICY?
First, let’s be clear on what AIDS policy is:
· Preventing infection by a retrovirus, HIV, that attacks immune system cells.
o Largely through education aimed at avoiding risky practices, such as:
§ Recreational drug use, particularly injection drug use.
§ Promiscuous sexual activity.
o Also through preventive measures (e. g., condoms).
o Advocacy, charitable and medical organizations are mobilized in support.
· Treating infected adults who have illness or symptoms with drugs.
· Development of drugs to attack HIV.
· Guiding infected adults not to have unprotected sex.
· Treating infected pregnant women with drugs.
· Giving newborns born to infected mothers chemotherapy to prevent infection.
· Encouraging people who participate in risky behavior (certain sexual or recreational drug practices) to be tested for HIV.
· Expanding HIV testing to the general public.
AIDS POLICY COSTS
Second, the cost:
· Close to $200 billion so far in federal funding.
o Three main costs:
§ Drug treatment (largely through Medicaid)
§ Research funding.
§ Advocacy group funding.
·
Just one state,
·
A
Research costs:
Expenditures are far higher per patient death than virtually any other disease.
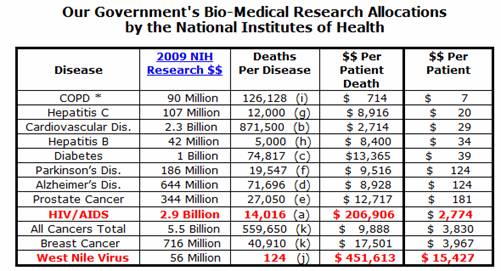
(data from
http://www.fairfoundation.org/factslinks.htm)
HOW IS THE POLICY DOING VERSUS GOALS?
Third, status versus goals (U. S.)
· Prevention of new infections.
o Number
infected in the
· Treatment of existing infections.
o Drug
treatments available in
o But median age at death remains under 45 years:

http://www.cdc.gov/hiv/topics/surveillance/resources/slides/mortality/slides/mortality9.pdf
o Patients with >200 CD4 T-cells/mm³ treated with combination antiretroviral therapy seem to have greater risk of non-AIDS illnesses than of AIDS illnesses.
(Guidelines for the use of antiretroviral agents in HIV-1-infected
adults and adolescents. Department of Health and Human Services. November 3,
2008; p. 21.)
· Education of public.
o Widespread.
· Vaccine {1}
o No. And a recent effort failed.
· Cure {2}
o None. Both HIV infection and AIDS are regarded as permanent.
TIME FOR AN AUDIT OR REEXAMINATION?
Clearly, lacking a vaccine and a cure, there is room for improvement.
However, criticism of AIDS policy goes far beyond such concerns, to include:
· The scientific basis for the policy
· The isolation and even the existence of HIV.
· The validity of the HIV tests.
· The usefulness and safety of drug treatments.
· Problems in drug development and drug trials.
That such concerns exist is troubling, considering truly enormous research expenditures and many thousands of scientific papers. It is particularly troubling that the critics include distinguished scientists, including Nobel Prize winners.
HIV TEST CONCERNS
First, “HIV test” is a misnomer – common tests only check for antibodies to HIV, not HIV itself.
Since AIDS is defined by (1) a positive HIV test, along with (2) one of some thirty or so conventional illnesses (such as pneumonia, wasting, cancer), the entire AIDS edifice thus rests on the HIV tests. So criticism of the tests has the potential to undermine the entire AIDS edifice.
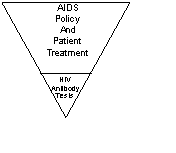
Figure 1 The AIDS edifice rests on the HIV antibody tests.
Some of the undisputed problems with the tests are:
- Disclaimers. {3}
All the test kits include disclaimers, which basically say, “Don’t rely on this test to diagnose your HIV infection”
- Checking for antibodies, not HIV. {4}
The usual tests do not check for HIV; they check for antibodies, assumed to be unique to HIV, which are produced by the body’s immune system, as an indirect indication.
- Varying criteria. {5}
Unlike any other disease test, the criteria vary widely by geography.
So someone who
tests positive in Africa might not in
- Difficulty in verifying results. {6}
Verifying a positive test (by finding actual HIV in the patient) is extremely difficult.
- Predictable variation by racial group. {7}
Professor Henry Bauer studied the database of HIV test results, and discovered that the percentage of positive test results is virtually always ordered as follows: Blacks test positive most frequently, followed by Hispanics, then Whites, and last Asians. This was true regardless of risk group.[1] Further, East Coast Hispanics (African-Caribbean ancestry) test like blacks, while those on the West Coast test like whites.
Suggesting that the tests may be measuring general immune system activity, rather than the presence of antibodies to a particular microbe.
Perhaps the biggest problem with the tests is the immutability of a positive result. {8} Once tagged, the person is basically stuck with the stigma of being HIV-positive forever, Because: (1) there is claimed to be no cure for AIDS (or way of getting rid of all HIV); (2) there is generally no way to prove or disprove the result by finding (or not finding) actual HIV in the person.
Despite these considerable problems and the life-wrecking consequences of a positive HIV antibody test, life insurance companies routinely require the tests, and authorities want to make testing even more routine, doing away with previously required signed informed consent forms.
WHAT ARE THE HIV TESTS?
Those noted above are the undisputed problems. There are, however, other problems, though not undisputed, that raise profound questions.
Before going into those, it will help to understand the different tests.
· The ELISA test is used for screening. Claimed to be highly sensitive (to avoid missing anyone who is infected), it can go overboard, and sometimes tags people positive when they are not. Nonetheless, in some countries it is the only test used.
o Oddly, for a condition whose traces are hard to find, the original ELISA test required that the samples be diluted by a factor of 400. {9}
·
The Western Blot test. Used in the
· PCR or “viral load”. While the PCR test claims to detect an actual part of HIV’s RNA genome (as opposed to antibodies to an HIV protein fragment), it is not allowed for use in determining adult HIV status. Its common use is to measure progress of drug treatment, by counting the number of HIV microbes, using the count of the RNA replications generated by the PCR in the sample as a marker for the amount of HIV.
Only the first two (ELISA and Western Blot) are normally used in determining HIV status. Those tests are claimed to be very accurate (99%+). Accuracy is claimed to increase by having samples retested, in the same way that the odds of heads or tails in tossing a fair coin will eventually approach the true odds if the coin is tossed enough times.
There are efforts to make HIV-testing routine in the general population. However, it can be shown mathematically that in low risk populations, even assuming claims of high accuracy for the tests, a large percentage of the positive tests will be false. See http://www.aidspetition.org/lowriskhivtests.htm for an explanation.
OTHER CONCERNS WITH THE HIV TESTS
With that background, here are a few less undisputed things some scientists and doctors have said about the tests:
- Validation. The HIV tests have never been validated by isolating purified, actual HIV from a patient testing positive. {10}
- The official validation goes back to the original 1984 work on HIV, which is acknowledged by the author as being inadequate for proof. {11}
- No subsequent validation has been done; rather, new test products are compared to previous test products. {12}
- When undiluted, previously negative patient samples tested positive on the original ELISA test[2]. Since ELISA apparently never detected a zero value, whatever it was detecting must be present in everyone, hence it could not be a valid test for the presence or absence of anything, including HIV. {13}
- Some of the proteins used in the Western Blot are claimed to be ordinary cell debris, not fragments of HIV. Even the co-discoverer of HIV, Luc Montagnier, believes that one protein in the test is just actin, a common constituent of cells. {14}
- Antibodies are not necessarily unique to a particular microbe or protein. An antibody can “cross-react” with a protein with a similar shape. {15}
- Many cross-reactions have been documented, including ones due to pregnancy, flu shots, and others. {16}
- Most AIDS patients also have other infections. Some say antibodies to those infections may be cross-reacting with the test proteins. {17}
- The “repeating tests of a patient’s sample increases accuracy” idea only works if there is no cross-reaction. Just as repeating tosses of a coin only works for a fair coin. If the false positive test is caused by a cross-reaction, retesting is likely to find the same cross-reacting antibody again. {18}
- Most infants who test HIV-positive (at nine months, when antibodies from the mother are gone), later lose their positive status, suggesting that they are either curing themselves, or that what the tests are detecting are not HIV antibodies. {19}
A CLOSER
LOOK AT HIV ANTIBODY TEST CONCERNS
Let’s look a bit more closely at some of the above.
- The accuracy claims for the tests are based on the assumption that antibodies to HIV are unique to HIV and its proteins. But items 11, 12 and 13, dealing with cross-reaction, indicate that assumption is wrong. If so, the accuracy claims would need, at the least, to be revised downward. Potentially, the entire strategy of relying on the indirect evidence of antibodies, rather than direct evidence of HIV, would have to be abandoned.
5. If the tests are, in many cases, detecting non-HIV antibodies, that could explain the anomaly of black people always testing positive more often than non-blacks, even in very low risk groups. One possibility is that black people, whose ancestry is in tropical climates rife with disease, developed very active immune systems that generate many more antibodies naturally.
3. Varying criteria. No one has ever explained why, unlike any other condition, AIDS and HIV use a diagnostic test whose criteria vary widely by geography.
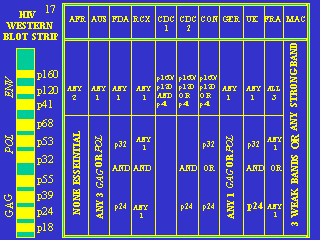
Figure 2 Chart showing varying criteria for a positive Western Blot HIV test in different geographic locations (See footnote for geography codes). (Original chart by Dr. Valendar F. Turner of The Perth Group of medical researchers). GAG, POL and ENV refer to several presumed HIV genes; The “p” numbers are proteins of various molecular weights. [3]
4. Difficulty in finding actual HIV in patients. Some would like to use the PCR test which, while it does not find actual HIV, claims to find one of HIV’s proteins. See discussion of the PCR later on.
- The self-curing babies are a potential coup de grace for the HIV tests and theory. If the tests are right, then HIV infection is capable of self-curing, contradicting a central tenet of AIDS science. If HIV is incurable, then the tests must be detecting something other than HIV, implying that millions have been misdiagnosed.
In response, HIV advocates contend that HIV antibodies, uniquely, somehow persist after the nine months when all other maternal antibodies have long disappeared.
MORE ON CROSS-REACTIONS AND DISCLAIMERS
According
to Dr. Philip Mortimer, Director of the Sexually Transmitted and Blood
Borne Virus Laboratory in the
"Diagnosis of HIV infection is based almost entirely on detection of antibodies to HIV, but there can be misleading cross-reactions between HIV proteins and antibodies formed against other proteins, and these may lead to false-positive reactions. Thus, it may be impossible to relate an antibody response specifically to HIV infection” (emphasis added).
It means
that if someone has a positive test we can’t be sure if it’s caused by HIV
infection.
The above
is from a presentation given in a court case regarding the HIV tests.[4]
CONCERNS ON VALIDATION OF THE HIV
ANTIBODY TESTS
Validation and Isolation
The most profound challenge to the HIV tests are items 6 through 8, dealing with the original validation of the tests.
The HIV antibody tests were originally developed and patented by Dr. Robert Gallo. They were initially used for blood bank screening, but were soon pressed into service for indirectly determining the HIV status of patients, in lieu of more difficult methods that would need to find HIV itself in the patients.
The FDA, is response to a query, cited three scientific papers as validation for the HIV tests: two by Gallo and his team, and one by Weiss (which relies on one of the Gallo papers).[5]
In response to a further query, the FDA acknowledged that no subsequent validation had been done.
So the inverted pyramid of AIDS policy, resting on the HIV tests, in turn rests on the slender base of two 1984 scientific papers, and one in 1985:
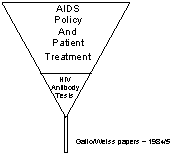
Figure 3 AIDS policy rests the HIV antibody tests, whose validation rests on three 1984-5 papers.
In one of Gallo’s papers, indications of HIV are
found in roughly 40% of patients. Much
later, Gallo acknowledged that would be insufficient to constitute proof. The Weiss paper found positive test results in
82% of the cases, using a single ELISA test without any confirmatory test, {20} although
Gallo acknowledged in court that the ELISA “gives too many false positives”.
These are obviously not slam-dunk results.
DETERMINING THE HIV PROTEINS TO USE IN
THE TESTS
To determine proteins to use in the antibody tests, it was first necessary to understand HIV’s genetic structure, or genome.
How did Gallo and his team determine the makeup of HIV, so that its proteins could be used for the HIV antibody test?
It took a while. The number of HIV proteins increased from just a handful to ten over a series of scientific papers.
As has been universally true, finding HIV using long-standard procedures proved difficult. So Gallo’s team grew patient serum in dishes, shocked the solutions with chemicals to break open the cells, and then looked for proteins whose size was characteristic of retrovirus proteins. An investigator, Neville Hodgkinson, described the next steps: “So, out of the 30 proteins, how did they select the ones to be defined as being from HIV? The answer is shocking, and goes to the root of what is probably the biggest scandal in medical history. They selected those that were most reactive with antibodies in blood samples from Aids patients and those at risk of Aids.” {21}
Mr. Hodgkinson’s article, in “The Business Online”, was thus titled ”The circular reasoning scandal of HIV testing.[6]”
ISOLATION AND PURIFICATION
The crux of the issue here has to do with what constitutes isolation and identification of a new virus or retrovirus.
For a regular virus, there is little difficulty in performing standard isolation and purification. For measles, take a blood sample, perform some procedures to isolate out non-viral material, and photograph the result under an electron microscope. See if what you have is uniform, and has appearance and other attributes characteristic of a virus. At that point, researchers can with confidence analyze the genetic makeup.
The same procedure works for retroviruses, other than HIV, as the picture shows.
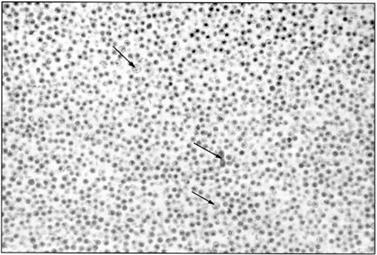
Figure 4 Electron microscope picture showing almost pure “type C” viruses (not yet called retroviruses in 1965). The three arrows point to contaminating debris.[7]
Gallo, instead, used the “grow in solution” approach. One rationale is that retroviruses, which insinuate themselves into the DNA of cells, are not floating around as much as other microbes, and hence are harder to find that way, although obviously some researchers found it possible for other retroviruses.
Asked in a television interview if he or Gallo had purified HIV, HIV co-discoverer Luc Montagnier answered in the negative.
A clear explanation[8] of the usual procedure for purification and isolation, and the problems with Gallo’s alternate approach, has been provided by Dr. Valendar F. Turner.
AFTER 14 YEARS, AN ATTEMPT AT STANDARD
TEST VALIDATION
Finally, fourteen years after the tests started being used, a research team attempted standard isolation and purification of HIV.
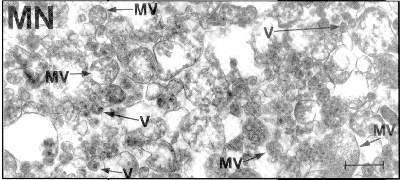
Figure 5 A 1997 attempt to purify and isolate HIV using standard procedures. Most of the content is contamination. The arrows point to particles thought to be HIV, which, however, seem to be too large to be retroviruses. [9]
The results were the obviously impure result in the photo; the particles said to be HIV turned out to be too big for a retrovirus. {21}
Some quotes may give a sense of the magnitude of this problem:
“… to date no HIV researcher has
published even one electron micrograph demonstrating the existence of even one
such particle in the blood of even one AIDS patient;”
Valendar F. Turner, M. D., in a court deposition.
"Up to
today there is actually no single scientifically really convincing evidence for
the existence of HIV. Not even once such a retrovirus has been isolated and
purified by the methods of classical virology."
Dr. Heinz Ludwig Sanger, Emeritus Professor of Molecular Biology and
Virology, Max-Planck-Institutes for Biochemy, Munchen.
Obviously, if
finding HIV in patients is so problematic, that reality calls into question the
validation of the HIV tests. True
validation must be against the “gold standard” of finding the actual retrovirus
in patients testing positive.
SIMILARITIES IN INFECTED AND NON-INFECTED
CULTURES
The same Bess research effort analyzed proteins found in presumably infected cultures (lanes B and C) and in uninfected cultures (lane A), using protein gel electrophoresis to sort the proteins by molecular weight.
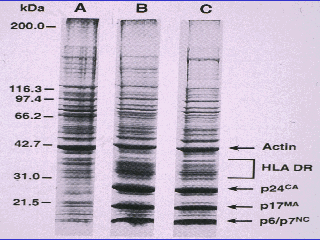
Figure 6 Protein gel eletrophoresis.
Dr. Valendar F. Turner, M. D., pointed out several problems with the slide:
“This slide proves a number of things:
1. If B and C are a virus then again it’s not pure because if it were none of
what you see A, which is just cells used to grow the virus, should be in Lanes
B and C.
2. Apart from the darker bands at the bottom of B and C there is no difference
between the three patterns. That’s the point. Although a few of the bands are
darker in B and C, because those proteins are present in a higher
concentration, they are still present in A, albeit faintly. So the only
difference between the virus and the cells used to grow the virus is the amount
of proteins. Not the types of proteins.
3. If B and C contain even impure HIV they should also contain all of the
proteins said to be present in HIV. But they don’t. Some are not there. There’s
no p41. But there is actin in the place where one would expect to find p41,
which proves Montagnier right. That p41 is actin. A cellular protein.
4. There’s also a protein where one would expect to find p120. Just above the
116.3 label. It’s not labeled p120 but if this is the p120 protein it’s also
present in the non-infected culture. Which does not have a virus. And if it’s
not the HIV p120 protein then the virus will be dead because without this
protein it cannot get inside cells to replicate.
5. So HIV proteins = cellular proteins and cellular proteins do not make a
virus.”
STILL MORE QUESTIONS
At this point, with many questions, few answers, goals still in the distance, and huge sums spent, any business would long ago have called an audit, just on the questions regarding the tests.
But the questions do not end there.
OTHER CONCERNS ON AIDS SCIENCE
Concerns on the scientific basis of AIDS policy.
These concerns are rooted in a simple fact:
· There is no scientific paper or collection of them among the many thousands produced that can serve as proof of the idea that sicknesses in people labeled AIDS patients are caused by HIV. {22}
The situation was not helped by the controversy surrounding the scientific papers which served to launch the policy, and which also later served as the basis for HIV testing.
Those papers, by Dr. Robert Gallo’s team, claimed to have discovered a new retrovirus which, however, turned out to have been sent to his lab by a French researcher, Luc Montagnier.
Gallo was subsequently cleared of scientific misconduct on
appeal, but the controversy ultimately had to be settled at the presidential
level between the
Gallo’s work had found indications of HIV in only 40% of his patients, which he has acknowledged would be insufficient as proof.
Nonetheless,
Virtually all research on other plausible causes of immune
system problems (drug use, malnutrition, environmental factors, etc.) was
halted, and funds were directed only to HIV-related research, along with
preventive education. {24}
The Nobel Prize?
Recently, the Nobel Prize for Medicine was awarded to Dr. Montagnier and team for discovering HIV, completely bypassing Dr. Gallo.
Dr. Montagnier’s work has a few puzzles, though:
· An interview in which he contended that neither he nor Dr. Gallo had succeeded in isolating and purifying HIV, despite what he called a “Roman effort”.
· He believes co-factors besides HIV are required for immune system damage.
· His seminal 1983 paper uses detection of reverse transcriptase (RT) as an indicator to show the presence of a retrovirus. But RT is not unique to retroviruses. (See the more detailed discussion of RT below).
· Dr. Montagnier has brushed off the need for pictures of HIV, but did show one in his Nobel presentation.
o However, the microbe lacked HIV’s characteristic “knobs” around the surface.
A more detailed and technical discussion of shortcomings in
the work by Dr. Montagnier can be found at http://www.theperthgroup.com/montagniernobel.html,
along with the pictures.
AIDS
SCIENCE AND STATISTICS
Once procedures existed for detecting what was said to be HIV in patients, first through the “grow in dishes” approach, later using the HIV antibody tests, epidemiology was used to understand disease patterns. Since AIDS illnesses, and positive test results, were found most often in a segment of the homosexual community, and secondarily in injection drug users, HIV was thought to be transmitted through bodily fluids.
So AIDS became an STD – a sexually transmitted disease.
AIDS was, at the outset, an STD that seemed to be largely restricted to males, occurring mostly in a segment of the homosexual community.
Strangely, though, when the Army first tested recruits, positive HIV tests occurred roughly evenly among young men and women.
While the definition of AIDS has now expanded to include
more female diseases (like cervical cancer), AIDS patients in the
For an STD, AIDS seems very hard to acquire. A study estimated the chance of infection from unprotected sex at around 1 in 1100 for male to female, and around 1 in 8800 for female to male.[10] {25}
Further, the spouses of many AIDS patients seem to be uninfected.
AIDS IS REDEFINED TO INCLUDE PEOPLE WHO
ARE NOT SICK
AIDS is rescued from statistical decline.
In the years just before 1997, AIDS cases had been leveling off, and seemed likely to decline. That year, AIDS was redefined to include people who were not sick - anyone with a positive HIV test, along with a low immune system CD4 cell count.
AIDS cases skyrocketed. {26}
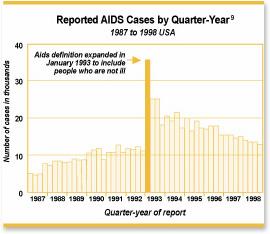
Figure 7 Quarterly AIDS cases (from Alive and Well: Rethinking AIDS).
HIV AND REVERSE TRANSCRIPTION INTO CELLS
Why did the original researchers think the particles they found were from a retrovirus?
Well, they found indications of an enzyme called Reverse Transcriptase, or RT.
A bit more background on cells and their makeup may help. Those familiar can skip.
A cell is a complex chemical machine, controlled by its nucleus which contains deoxyribonucleic acid, or DNA. The DNA can be thought of as a blueprint, used to make copies of sections of the blueprint through a process called “transcription”. Those copies are made of RNA, a kind of complement to DNA, and are then used to assemble a needed protein or set of proteins, in a process called “translation”.
DNA forms a helix, like the two sides of a twisted ladder, linked together by many pairs of nucleotides, analogous to the steps joining a ladder’s sides, abbreviated by the letters C, G, A and T. Chemically, C can only pair with G, and A can only pair with T.
During transcription, one “side” of a portion of the helix unwinds from the other, and is used as a template to build a complementary RNA molecule. Unlike DNA, the RNA molecule only has one “side”, with each half step being the complementary nucleotide. C in the DNA molecule will result in G in the RNA molecule, and vice versa. Similarly T results in A in RNA. A, however, results in a slightly different nucleotide than T, abbreviated by U.
So
a segment with TCAACGTG
||||||||
AGTTGCAC,
On
unwinding, would look like TCAACGTG, matching with:
--------
AGUUGCAC (RNA
molecule)
The RNA molecule then leaves the nucleus, going to the cell’s cytoplasm, where translation into the required proteins takes place.
In higher organisms, there are extra sequences intermixed in the gene segment, called introns, which are removed from the RNA before translation.
Because they are complementary, RNA and the DNA segment contain the same information. Given one, it is possible to reconstruct the other.
Retroviruses, unlike regular viruses, have their genetic material in the form of RNA, not DNA. The retrovirus uses Reverse Transcriptase (RT) to insert itself into the cell’s DNA, so called because the process is the reverse of the usual DNA to RNA transcription.
The original HIV researchers regarded the presence of RT in the solution as evidence of the presence of a retrovirus.
REVERSE TRANSCRIPTASE NOT UNIQUE TO
RETROVIRUSES
RT, however, is not unique to retroviruses. {27}
As things turned out, cells are more complicated than imagined. It happens that there are normal processes that use RT. So RT was not a sure sign that the dishes contained retroviral particles.
In fact, even more surprising, the belief that heredity is only encoded in DNA seemed to be disproved by 2006 experiments[11] that indicated RNA can play a role, too.
Perhaps we did not understand RNA-containing things, including retroviruses like HIV (when outside the cell), as well as we thought.
THE ELUSIVE RETROVIRUS
If HIV damages the immune system, one would expect many such cells to be found. But very few seemed to have HIV, a number that the body could easily replace. {28}
Researchers sought an explanation, and Dr. David Ho came up with a model to explain the anomaly.
Later, other researchers contended that his model was mathematically unsound.
MEASURING HIV INFECTION – THE PCR TEST
AND ITS CONCERNS
If a patient is infected, doctors would like to know to what extent, and both doctors and drug companies would like to know if treatment is improving things.
Thus there was great demand for a way to measure the degree of HIV infection, ideally by counting the number of HIV microbes in the patient.
As has been discussed already, isolating actual HIV from the patient is very hard, foreclosing an approach that would count microbes from a sample under the microscope and project that count to the entire body.
Earlier, Kary Mullis had won a Nobel Prize for the discovery of the PCR technique. The PCR can cause a target molecule sought in a sample to be replicated many times until it becomes detectable.
So researchers seized on the PCR, using what is said to be part of HIV’s RNA genome as a stand-in for HIV, and treating the number of replications as a measure of HIV microbes.
There are several concerns:
1. The PCR test is not licensed for diagnosis (in adults).
2. The PCR detects only a part of what is said to be HIV, not HIV itself.
3. Questions on the validation of the PCR, and the existence of RNA in people due to exposure to toxins.
4. PCR test results can vary widely for the same sample.
5. Dr. Mullis, creator of the PCR technique, doubts that HIV causes AIDS, and disagrees with the use of the PCR for quantitative measurements.
6. Recently, researchers discovered that PCR test results did not correlate with immune system status[12], calling into question the value of the test in measuring HIV infection. {29}
WHY USE THE PCR FOR INFANTS, AND NOT
ADULTS?
Recall that infants, up to 9 months, carry maternal antibodies of all sorts. So if the mother was infected with HIV, and thus had HIV antibodies, the child would, too, even if the infant was not infected by HIV.
Thus the usual HIV antibody tests, if given to an infant before 9 months, might register infection falsely.
So officials needed to detect HIV directly.
But that cannot readily be done.
So the PCR was used as a substitute, despite the problems listed above.
A physician and researcher, Dr. Valendar Turner, asked the CDC why the test was approved for infants, but not adults. The question could also have been asked in reverse: why approve it for infants if it is not approved for adults. It should be, after all, the same entity in both cases.
Despite great efforts at follow-up, and a promise by federal officials, no answer was ever supplied. {30}
AIDS IN
Once having a test (ELISA, Western Blot) that was said to detect HIV infection (via antibodies), and given the belief that HIV could damage the immune system, researchers checked various populations with illnesses.
One of those populations was in
Moreover, those said to have AIDS (one of the some thirty
diseases in the definition, plus a positive HIV antibody test), were generally heterosexual
and of both sexes, a different pattern from the
Typical diseases in
Once tagged as an AIDS statistic, the patient is advised not to have unprotected sex. Since unprotected sex is necessary for conception, those Africans are being told, effectively, to be childless, terminating the future families of some twenty-five million people. {31}
AFRICAN AIDS AND SEX (?)
How do Africans catch HIV and come down with AIDS?
Well, remember that AIDS is believed to be an STD. Some researchers cast an eye at truck drivers
roaming
Eventually, a team of conventional British AIDS researchers
decided to do a rigorous study of how HIV was spread by sex in
Their report[13] shook the AIDS establishment. The researchers concluded that most AIDS cases could not be accounted for by sexual transmission. {32}
CONCERNS WITH AIDS DRUGS
The basic difficulty in combating viruses (or retroviruses) is that they are found in and use the body’s cells. Targeting viruses can wind up targeting the cells themselves. Viral infections in the past were treated by building up the patient so his or her immune system could fight off the infection.
On concerns, one can point to Dr. Robert Gallo, who told an
AZT’s side effects include ones “similar to HIV disease”.
AZT is a carcinogen and mutagen.
AZT was the original AIDS drug, a failed cancer chemotherapy that was urgently pressed into service to provide some way of treating AIDS patients.
AZT effects cells, and was chosen as a way of getting at the HIV imbedded in cell’s DNA. The downside is that normal cells can also be affected.
Lab samples of AZT, in quantities less than initially prescribed amounts, carry stringent warnings to avoid contact.
Officials pressed for approval of AZT despite concerns of the government review panel.
Not long after AZT use began, a large study, the Concorde Study, showed that AZT was not effective in people who were HIV-positive, but still healthy.
Nonetheless, it continued to be prescribed. It was even recommended for pregnant women. One study documented cases of birth defects among babies born to women so treated[15].
AIDS drugs like AZT work by inhibiting DNA replication, a
necessary process for normal cell growth and replacement. The latter point is a particular concern when
such drugs are administered to pregnant women and infants, where cell
replication and growth are critical to normal development.
The DNA terminator drugs were supplemented with protease
inhibitors, which were intended to affect an enzyme required for retrovirus
replication. However, there are protease
chemicals in the body which are essential to life which run the risk of being
affected, even though they are not the target.
Another class of drugs acts to inhibit reverse transcriptase
(RT). Since it is now generally
acknowledged that RT is also used in normal cellular functions, such drugs have
the potential for severe side effects, as the Physicians’ Desk Reference item
on Viramune (nevirapine) notes.
AIDS DRUGS EVEN FOR THOSE NOT SICK
Dr. David Ho proposed the “cocktail” treatment, combining several drugs with the objective of getting at HIV in multiple ways.
Some critics contended that improved results were largely
because the amount of AZT (or similar drugs) given to patients was greatly reduced
in the cocktail.
To suppress the spread of HIV, Dr. Ho proposed giving the treatment to patients with HIV who were not sick.
Subsequently, the government acknowledged that doing so was a mistake, and recommended that drugs not be prescribed to patients who were not actually sick[16].
SUFFER THE INFANTS ……
To prevent transmission of HIV from mother to child, officials recommended treating the mother with powerful AIDS drugs to minimize the number of HIV particles, and thus transmission.
Despite past views that cautioned against even giving pregnant mothers aspirin.
These drugs, such as nevirapine, are powerful drugs that affect cell replication, which is what infants do to grow and develop. {34}
After delivery, in cases where the mother was HIV-positive, the newborn is given similar powerful AIDS drugs during the first weeks of life to reduce the odds of infection.
A Reuters article, January 4,
2001, indicates the powerful side effects the drug can have:
The Centers for Disease Control and Prevention (CDC)
said it found 22 reported cases of serious side effects among people who took
the drug fearing exposure to HIV, the virus that causes AIDS, because of a
needlestick or similar injury.
Dr. Elise Beltrami of the CDC's National Center for
HIV, STD and TB Prevention said the side effects have included liver toxicity
and severe skin reactions. In one case, a 43-year-old health-care worker needed
a liver transplant after suffering liver failure.
Nevirapine, sold under the name Viramune, is approved
for use as an antiviral drug for people with HIV infection. It is also used to
prevent transmission of HIV from a pregnant woman to her child.
However, the CDC said that because most occupational
exposures to HIV do not result in transmission of the virus, the risk of side
effects from nevirapine should be balanced against the risk of HIV
transmission.
"In this setting, the risk of HIV transmission is
very low and, in most cases, the risk of taking nevirapine would outweigh the
risk of using it for possible prevention of HIV,'' Beltrami said.
The drug's manufacturer, Boehringer Ingelheim/Roxane
Laboratories, Inc., notified health professionals in November that it was
strengthening product package warnings because of continued reports of
"severe, life-threatening and in some cases, fatal hepatotoxicity.''
Beltrami said the adverse reactions reported among
health- care workers "do not, in any way, apply to the use of nevirapine
in other settings.''
A front page article in the
April 29, 2007 New York Times described the memory loss / cognitive deficits
that can occur in cancer chemotherapy patients.
Newborns and infants are given powerful drug treatments if they test positive
on the HIV antibody tests.
Could such treatments wind up affecting the mental abilities of the babies?
Something to ponder as our government encourages AIDS drugs for pregnant
mothers and their newborns.
More humility, given the extraordinary complexity of the human mind and body,
would be welcome in those who propose such treatments.
SUFFER OLDER CHILDREN AS WELL …..
How can new AIDS drugs be tested?
Well, some with AIDS illnesses might consent to volunteer.
But there was another group that could be volunteered without their direct consent: HIV-positive or AIDS patient children in institutional care. The institution could volunteer them, with the rationale that the drugs might help them.
Legally, such children must have an independent “patient advocate” to look out for the child’s interests, if there is no parent.
But according to an investigative article[17], children in one institution were enrolled in drug trials without that patient advocate, and in some cases without consent of the actual parent.
The drugs are many, and hard to take. Some children resisted, remedied, according to the investigative article, by surgically inserted tubes to deliver the drugs regardless. {35}
The article forced a
QUESTIONS ABOUT AFRICAN DRUG TRIALS ON INFANTS AND MOTHERS
Nevirapine was tested in
In some cases, death or severe problems resulted.
HIVNET 012, nonetheless, was used as the basis for
government programs distributing nevirapine for use in
MONEY, BUT NO QUESTIONS
This is the policy that many advocacy and charitable organizations have embraced.
It confers lifelong stigmatization, cures no one, enriches drug companies, can have severe side effects on those treated with drugs, and has many questions and problems, most without good answers.
The policy does have the effect of scaring the bejabbers out of young people tempted by sexual promiscuity.
And it showers money on organizations that promote the policy.
As long as no questions are asked.
No questions allowed …
Professor Peter Duesberg found that out when he raised hard to answer questions about AIDS policy, and found his research grants eliminated, and his previously stellar scientific career road-blocked. {37}
AIDS officials hammered the point home, calling for those who question the theory that HIV causes AIDS to be jailed[18]. {38}
Truth and silence are usually
incompatible.
The silence in the face of all the problems with AIDS policy is such a case.
- Condemning millions to lifelong stigmatization, erasing the future families of tens of millions,
- Offering no hope other than risky, continual, and ruinously expensive drug treatments.
Surely we are better than this …
…. Aren’t we?
WHAT IS NEEDED
1. Answers to open questions (or acknowledgement that there are no answers).
2. Open hearings that listen to a wide variety of views and critiques.
3. Legislation to cause open issues to be resolved.
There would be no pre-determined outcome. It may be that current policy cannot be improved; or perhaps it is sound, but can be improved somewhat; or, the policy may be fundamentally flawed. That last possibility is the troubling one, the possibility that AIDS policy has been a very large mistake.
But if it is mistaken, the policy should be corrected urgently for the sake of those affected.
We really ought to find out for sure before implementing policies with such profound impacts on our fellow human beings.
Our website is aidspetition.org.
References